Decoding LIS vs LIMS: Which One Suits Your Lab’s Needs?
Despite having the same overall objective—efficiently handling laboratory data—they have diverse functions and features that are tailored to the requirements of various laboratory types. To assist you in choosing the best solution for the needs of your laboratory, we will examine the distinctions between LIS and LIMS in this blog.

Struggling with Managing Complex Lab Operations? Transform Decision-Making with Our AI-Based Clinical Decision Support System Software Solution

“The expansion of molecular diagnostic techniques, including genomics and personalized medicine, and the need for automation and robotics in laboratories primarily drive the market growth. LIS helps in automating various laboratory processes, such as sample tracking, result management, and data analysis, leading to improved productivity and accuracy. Additionally, the growing need for integrated healthcare systems and the rising adoption of electronic health records drive the demand for LIS.”– Markets And Markets
What is a LIS: Overview
Clinical and medical laboratories are the main users of LISs, which are specialized software platforms. To ensure smooth interaction with electronic health records systems, an LIS system in lab is made to store patient data, test orders, and results. Similar to a LIMS, a LIS keeps and oversees lab data. But the purpose of a LIS is to store:
- Results reporting and delivery
- Patient data management
- Billing and insurance processing
- Compliance with regulatory standards (e.g., HIPAA, CLIA)
- Test ordering and scheduling
A LIS system in lab is crucial to medical lab workflow optimization, allowing for quicker and more precise diagnosis and treatment choices.
What is a Laboratory Information Management System?
High-volume samples and related data points can be managed by non-medical testing facilities using laboratory information management system software (LIMS). As a result, a lab can get accurate outcomes and automate processes like tracking samples. To streamline laboratory operations, improve data integrity, increase productivity, and ensure regulatory compliance, a laboratory information management system does exactly what its name suggests: it manages enormous volumes of data at different stages of lab operations (such as sample management, workflow management, inventory management, etc.).
Since it facilitates laboratory workflow management while abiding by established rules, a laboratory information management system (LIMS lab management software) is extensively utilized in a variety of regulated industries. A few examples of LIMS systems are as follows:
- To track hazardous products, manage chemical inventory, and ensure adherence to safety standards, a chemical laboratory uses a LIMS.
- LIMS is used by a food and beverage laboratory to oversee food safety testing, monitor ingredients, and ensure compliance to food safety regulations.
 LIS vs LIMS: Top 5 Benefits of LIMS Over LIS
LIS vs LIMS: Top 5 Benefits of LIMS Over LIS
Although LIS and LIMS are both essential tools for laboratory administration, LIMS has several extra advantages that make it especially appropriate for industrial, research, and quality control labs. The following are top benefits of LIMS over LIS:
1. Advanced Workflow Automation
- LIMS: Automates complex procedures such as sample tracking, testing, and reporting, for areas like environmental testing, food and beverage, and medicines. LIMS enhances non-clinical lab efficiency by supporting more complex and varied workflows.
- LIS: Focuses on workflows for diagnostics, including patient reporting and testing.
2. Customizable Reporting & Analytics
- LIMS: Provides configurable dashboards and comprehensive reporting tools for examining trends, performance indicators, and operational effectiveness. For labs seeking to enhance decision-making and streamline procedures, LIMS offers greater insights.
- LIS: Emphasizes diagnostic reports for medical professionals.
3. Comprehensive Data Integration
- LIMS: Connects to third-party software, enterprise systems (such as ERP and CRM), and a variety of other tools. By linking to a wider range of tools and systems, LIMS improves data flow between businesses.
- LIS: Primarily connects with medical systems, such as electronic health records, or EHRs.
4. Enhanced Traceability & Audit Trails
- LIMS: Offers thorough audit trails for each test, sample, and outcome, ensuring complete accountability and traceability. For labs needing thorough documentation for audits and regulatory inspections, LIMS is more appropriate.
- LIS: Keeps track of test results and patient information, but it is less concerned with sample-level traceability.
5. Support for Non-Patient-Centric Data
- LIMS: Maintains data for samples such as industrial products, chemicals, raw materials, and environmental specimens. For labs that need strong data management capabilities and handle non-clinical samples, LIMS is perfect.
- LIS: Emphasizes diagnostic results and patient data.
Are There Any Crossover Features Common to LIS vs LIMS?
- LIS system in lab is now more than just a patient information management system. Test results and sample information related to a patient can be managed by a LIS. An LIS can currently import data from local databases, software, instruments, and other sources, just like LIMS. From the standpoint of a clinical lab, more flexible workflows are made possible by the ability of more recent LIS systems to report test results and track sample placement.
- Like LIS, LIMS systems, which were formerly restricted to patient sample handling, are now providing thorough auditing for patient testing. Ensuring staff accountability requires the whole chain of custody to be recorded for patient samples. Because users are interested in who updates or deletes a record in addition to who accesses it, LIMS systems have also adopted this.
- A LIS system’s billing management feature is making its way into a LIMS system, particularly in private healthcare institutions. Modules for billing and CRM (Customer Relationship Management) assist in organizing and managing client data and accounting.
- There is no denying that LIMS and LIS share many characteristics. Along with meeting other laboratory needs, LIMS’s function is to effectively handle clinical trials, biobanks, and veterinary diagnostic lab procedures. It’s important to note that these LIMSs’ features frequently show a lot of similarities to those of LISs. Both systems can record clinical test findings, retain patient demographic information, and make it easier to comprehend the results.
This convergence highlights LIMS systems’ innate flexibility, which allows them to adapt to a variety of workflows and successfully handle everyday challenges that arise in laboratory operations.
Eliminate Operational Bottlenecks & Improve Compliance with NextGen Invent’s Digital Healthcare Software Development Services.
LIS vs LIMS: Understanding the Differences and Choosing the Best Fit
It used to be simpler to determine if your lab required an LIS vs LIMS. The differences are becoming increasingly hazy as functionality moves between the two systems.
The primary difference is that a LIMS was created for batch testing, frequently in situations where the names of individual patients are insignificant, whereas a LIS was created to handle individual patient records. Data tracking, instrument interfaces, and the effectiveness of workflow management are currently supported by both LIS and LIMS. To support testing accuracy, a laboratory manager can set up maintenance plans for analyzers or equipment and calibration date reminders with the aid of both LIS and LIMS.
Although it is now more difficult to distinguish between the LIS vs LIMS, their original development goal is still the same.
The fact that some phrases, like “clinical” and “workflow,” are used to refer to the two systems in different ways contributes to the misunderstanding. While “clinical” in the LIMS typically refers to clinical trials including the testing of novel medicines, “clinical” in the LIS refers to the treatment of patients. Distinct laboratory testing paths—patient versus research study—lead to distinct information systems for workflow.
Optimize Patient-Centric & Research-Driven Workflows Seamlessly with Our Scalable AI-Powered Healthcare Solutions.
LIMS vs LIS: Making the Right Choice
Your laboratory’s unique demands and requirements will determine whether you choose LIS or LIMS. Consider the following factors:
Choose LIS If
- You manage a clinical laboratory that specializes in diagnostic and medical tests.
- You require a system that can handle patient information, such as orders and outcomes.
- You need a system that complies with legal requirements like HIPAA and CLIA.
- You’re searching for a system that is less complicated and more straightforward.
Choose LIMS If
- You manage a laboratory for production, quality assurance, research, or development.
- You require a system capable of tracking, analyzing, and reporting sample data.
- You need a system that complies with regulatory requirements like FDA and ISO.
- You require a system that is extremely adaptable to your unique laboratory requirements.
- You want a system with additional features and advanced capabilities for data analysis and visualization.
The choice between LIS vs LIMS will ultimately be based on your laboratory’s unique demands and specifications, as well as your financial situation and available resources. It’s critical to thoroughly weigh your options and choose a system that can accommodate the particular requirements and objectives of your laboratory.
Conclusion
Choosing between an LIS and a LIMS ultimately depends on your lab’s unique operational needs, regulatory requirements, and future goals. While LIS focuses on clinical workflows and patient-centric data, LIMS offers broader capabilities for managing research, testing, and operational efficiency. Understanding these differences is crucial to making an informed decision that optimizes both performance and compliance.
At NextGen Invent, we go beyond traditional LIS and LIMS capabilities with ours artificial intelligence-based clinical decision support system. Designed to empower labs with advanced analytics, real-time insights, and intelligent automation, our services bridge the gap between data management and actionable decisions. Whether you’re managing patient diagnostics or complex research workflows, our AI-powered platform helps accelerate decision-making, improve accuracy, and enhance patient outcomes.
Ready to future-proof your lab? Discover how NextGen Invent’s clinical decision support system software solutions can transform your lab operations. Stay tuned for Part 2 of this blog, where we’ll dive into how we’re solving RCM, results reporting, and clinical decision support challenges for labs and healthcare providers.
Frequently Asked Questions About LIS vs LIMS
Related Blogs
The Crucial Role of Laboratory Management System in Modern Clinical Labs
Does your lab have ongoing difficulties meeting the demands of patient diagnostics? Are out-of-date systems and mistakes made when entering data by hand slowing down your team and affecting patient care?
The Advancing Role of Generative AI in Clinical Trials
Generative AI has the potential to not only alleviate the growing expenses linked to clinical development but also bring about a paradigm change by accelerating work throughout the whole clinical lifecycle.

Struggling with Staffing Shortages? How Lab Automation Can Solve the Crisis!
The lack of lab personnel has been an issue for many years and has now reached a critical stage. An estimated 20,000 to 25,000 laboratory technologists are needed nationwide, with vacancy rates as high as 25% in some US regions.
Stay In the Know
Get Latest updates and industry insights every month.
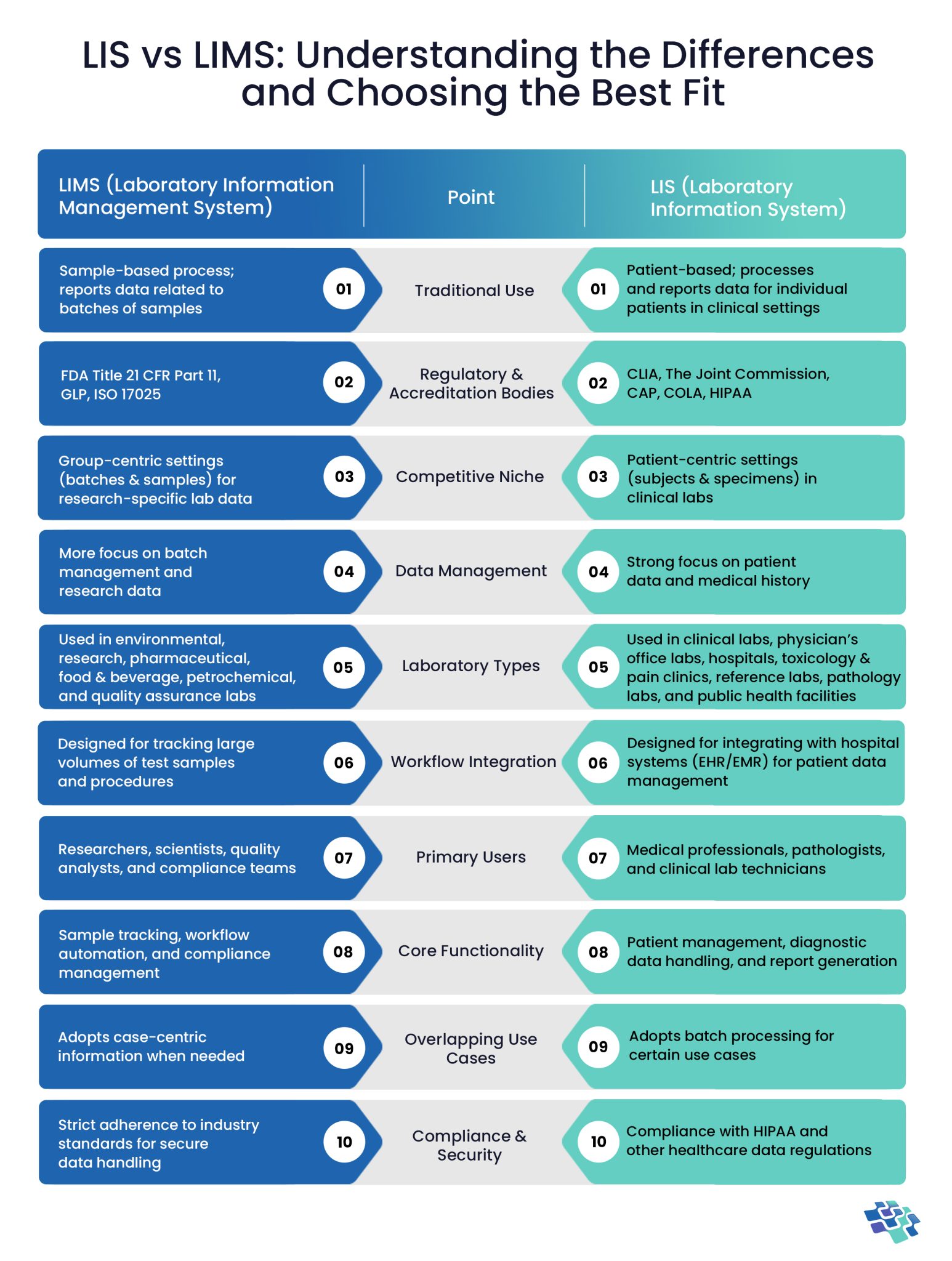 LIS vs LIMS: Top 5 Benefits of LIMS Over LIS
LIS vs LIMS: Top 5 Benefits of LIMS Over LIS